Our customer manufactures pharmaceutical products for veterinary use, which implies that the dossier had to be treated in accordance with Good Manufacturing Practices, in particular regarding works on automation and computers.
The first part of that work on a IMA SENSITIVE 350 used labeler (year 2014) consisted in increasing drastically the format capacity (some of the formats were even outside the limits of the initial equipment), in terms of diameter, number and geometry of the vials and labels. Besides, a security audit was realized, following the intervention of an approved inspection agency. This conducted to the reinforcement of the security chains, along with casing and securing of the risk zones for the operators. New setups resulted in the creation of new receipts of production, related to new formats, and in the optimization of the receipts of other existing formats.
- Refurbishment and enlargement of the main conveyor. Overhaul of the mechanical parts and replacement of the carrying belt.
- Removal of the products’ quality control sample-taking line.
- Modification of the output and storage of the rejected non-conform products. Adaptation of the ejection system to any type of vials.
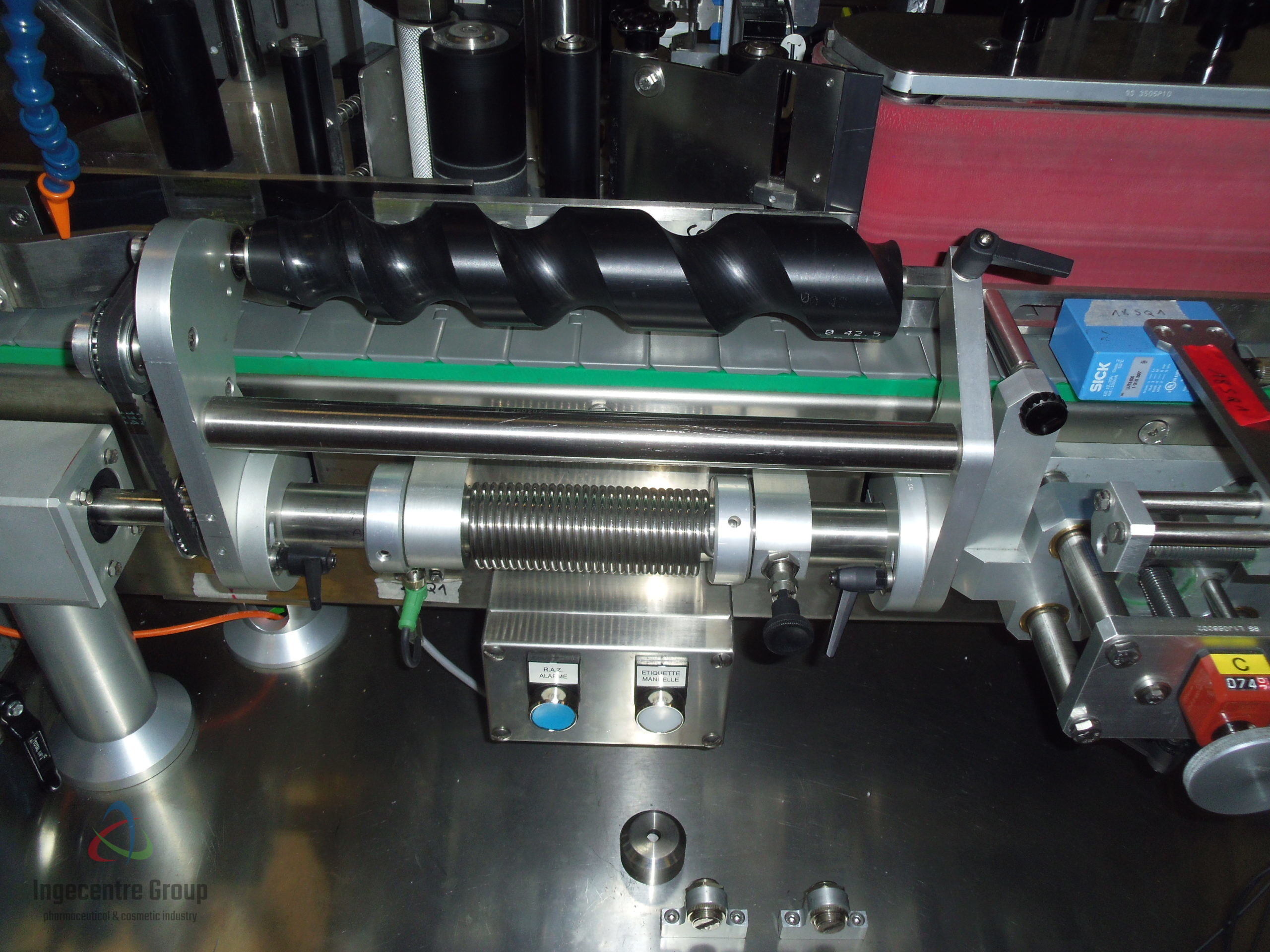
- Study and realization of the feeding and spacing screws, specific to the new dimensions of the formats. Modification of the transmitting/pressing device of the spacing screw.
- Improvement in ergonomics and in the implantation of some formats’ adjustment areas.
- Study and realization of the modifications of the casings and doors, in order to adapt to the new products’ dimensions.
- Study and realization of new safety adaptations, with addition of sensors on casings’ security chains.
Integration of emergency buttons to the compressed air supply’s cut-off system.
- Improvement of the label tape’s guidance, eliminating production breaks completely.
- Adaptation of the controlling system to new conditions of functioning (including operator messages and alarms).
- Creation of suitable receipts for the new formats.
Adaptation of the automatisms to the new formats, particularly concerning the control terminal.
Optimization of existing receipts, leading to a sharp increase of the OEE, because of a higher speed than before, and of the quasi-complete elimination of belt’s breaks.
- Re-putting the used labeler in line, coupled with an upstream turning accumulation table.
Putting into service again, and tests.
- Tests with all the vials/labels combinations asked by the customer, in collaboration with the person in charge of the qualification. Tracking of the results.
Creation of a complete matrix of the parameters and setting dimensions, for each format realized.
- Providing of a complementary technical documentation, describing in detail the modifications realized, the new functionalities and the new conditions of exploitation:
- Instructions manual (use, maintenance, format change …).
- Update of the electrical and pneumatic diagrams.
- Technical documentation of the new components.
- Matrix and recommendations for formats’ settings.
- Program and application program of the operator terminal (new version).
- Tracking of the modifications of Functional Specifications (MIPCO being not able to supersede the manufacturer).
- All the changes concerning elements impacting the controlling system were tracked in detail, for integration and taking into account during the operations of qualification of the equipment.
The monitoring of the results of MIPCO’s work lets appear a sharp amelioration of that used labeler in matters of productivity and security.